How to make glowing water beads: fun fluoro science for kids.

OMG, I just made GLOWING water beads. For reals! (Haha, I’m totally high fiving myself x 10 over here.)
You see, ever since we made glowing slime a few months ago, our black UV light torch has been sitting on top of our fridge, taunting me to come up with some more play ideas that glow or fluoresce.
My first thought was to send the kids to search for urine stains on the carpet – but given that we have a new puppy and a toilet training toddler, I thought that might not be the biggest challenge.
And then I thought about how the kids have been going nuts about water beads all summer long, and I thought…. I wonder if I can make glowing water beads??? Wouldn’t that be awesome!

And it worked. 🙂
Sure you need a black light, but they do really and actually glow.
My husband (Mr Banya) didn’t believe me at first. He reckoned that anything would glow under one of ‘those’ lights. (The black light used for all of these pics is this LED torch that I bought last year.)
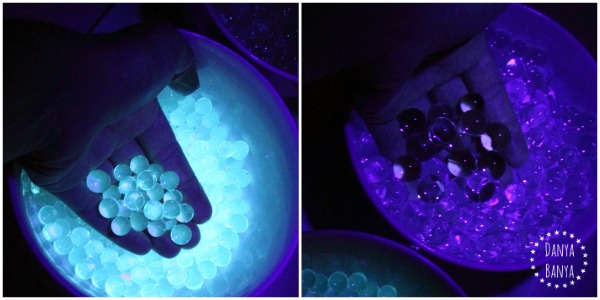
So I decided to do a little experiment: my ‘glowing’ water beads in the left bowl, and plain Jane water beads in the right bowl.

And I think I convinced him to change his mind. 🙂
To the human eye, the black light gives of a purplish light, which you can faintly see reflecting off the normal waterbeads on the right. In comparison, the glowing waterbeads have a really strong fluorescence, so much so that they no longer look transparent. They look like they are shining from within, rather than just reflecting light from the outer surface area.
Cool….
Here’s how the look against my hand – glowing water beads on the left, normal water beads on the right.
So, in case you are wondering
how to make glowing water beads
It couldn’t be simpler. All you need is:
- 1 packet of clear, non-toxic water beads. (I bought mine here, although they look similar to these ones
.)
- 2 cups (or thereabouts) of tonic water (make sure that quinine is listed as an ingredient).
- an ultraviolet (UV) black light. (There are many types, so have a look around. We have this LED torch
, and I also want to get a bar one
at some stage.)
Soak the water beads and tonic water together in a bowl. Let sit until maximum absorbency and strain. Turn off the lights, and turn on the UV….
And then you have yourself some awesome glowing water beads. Haha, how’s that for easy!

Interesting to note:
- I’m not sure exactly why (I believe it may have something to do with osmosis), but water beads soaked in tonic water, even at maximum absorbency, are significantly smaller, and slightly yellowish in daylight when compared to regular water beads. They are also sticky to touch, because of sugar content of the tonic water.
- Quinine is commonly used for various medicinal reasons, however it is also an ingredient (in minute quantities) of most brands of tonic water, to give a distinctive bitter ‘tonic’ taste.
- Fluorescence, in this instance, occurs because the quinine absorbs ultraviolet (UV) light, which is invisible to the human eye, and emits it back at a visible wavelength.
- Quinine is highly fluorescent, even in low quantities. It is so consistently fluorescent, that it is used in photochemistry as a ‘common fluorescence standard’.

I use activities like this to introduce scientific concepts to my daughters (JJ is 4.5 and Bee is 2.5 years old) in a fun and hands on way. It doesn’t matter that they don’t understand the theory behind it at this point: it’s enough that they can see it, and touch it, and be amazed. They are learning to associate science with fun and wonder. Using words like absorb, transparent, fluorescent, ingredient, quinine and ultraviolet light in context increases their familiarity with these words, and because I favour using scientifically accurate vocabulary in our play, they quickly grasp the meaning and start using these words themselves.
What other things might be fluorescent under an ultraviolet light? Experiment and find out. Finding out that something doesn’t fluoresce is just as useful in testing a hypothesis, as finding out that something does. (You should be able to find quite a few things that glow under a UV light around your home – white paper and teeth are good ones. Or you try looking in your wallet, and for something that you might use to brush your teeth).

Whenever we do activities like this with my daughters, we fistbump “this is science” and chat about how important it is to ask “I wonder how that works?” when we look at the world around us. Then we proclaim ourselves “Science Girls”. (They are lapping up the science love right now, and I really hope that they can keep their sense of wonder and inquiry: it’s this that makes a true science girl, regardless of her chosen profession.)
If you are after some more science fun, you might also like (click on the image to go to the full post):
Or for more water bead play:
{Please note that the water beads I buy are non-toxic, which means they are safe for kids to touch, but are not edible in either the unexpanded or expanded form. My two year old is no longer in the mouthing phase, as is well-supervised when playing with water beads. Please use your best judgement when introducing young children to small objects. Unexpanded water beads could expand in the stomach. Expanded water beads are a choking hazard. All activities on Danya Banya require attentive adult supervision at all times. If you are uncertain whether this activity is appropriate for the children in your care, please see your paediatrician for further advice.}
If you have children who still like to put things in their mouths, you might like to check out Fun At Home With Kid’s edible glowing water beads instead).
xx Danya
* This post contains Amazon affiliate link(s). An affiliate link means I may earn a commission if you make a purchase through my link, without any extra cost to you. It helps to keep this little blog afloat. Thanks for your support.








#
These are cool. I am a girl of the 80's so anything that glows is alright with me.
#
Haha, I may have just worn fluoro socks growing up too. 🙂
#
Have you tried with diet tonic water? Just wondered if it might reduce that sticky factor you spoke of….love the idea, though. Thanks for sharing!
#
No, but I like the way you are thinking! Might have to try that…..